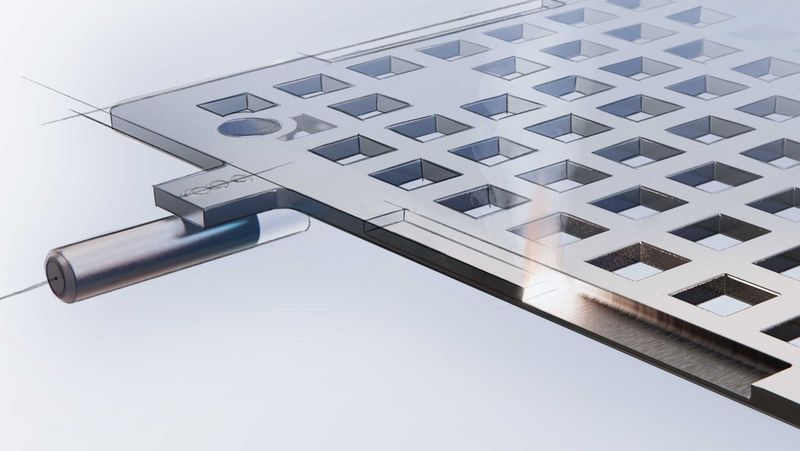
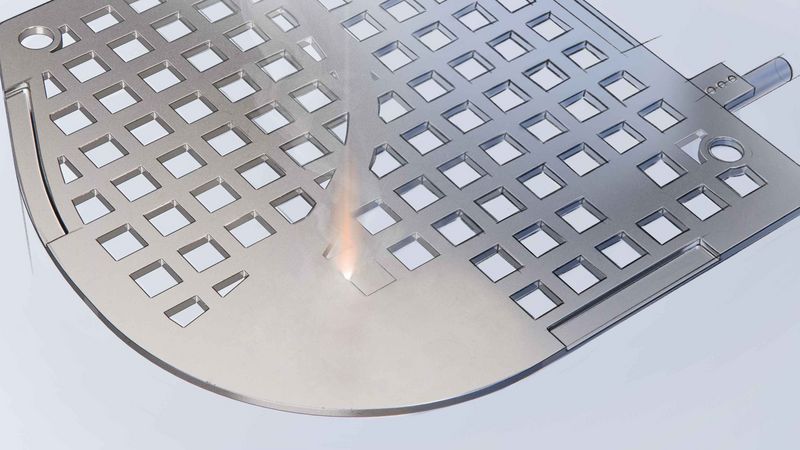
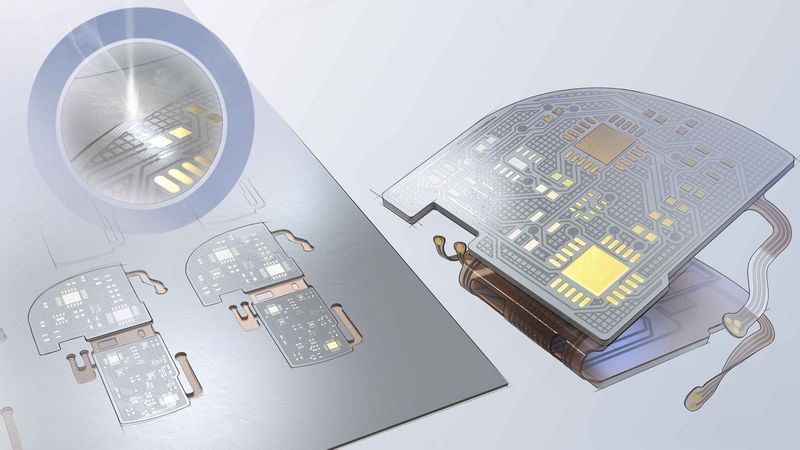
Implantable medical devices such as pacemakers consist of tiny components and sensitive electrical components. In the human body, these devices must function without problems and not cause any harm in the process. The devices are subject to a wealth of technical requirements and therefore place high demands on production processes and equipment: since the components used are often very delicate and the production steps very complex, many manufacturers rely on manual labour to assemble the implants. On the one hand, this is problematic because it is becoming increasingly difficult to find qualified employees. On the other hand, it makes it difficult to expand production, especially with additional production sites. In addition, manufacturers are required to document all production steps in a traceable manner.
Since implantable medical devices are vital for many people, they must be available in sufficient numbers. To ensure this, production systems need to function reliably, have minimal downtime, and enable fast (maintenance) service. In this context, it is becoming increasingly important that production capacities and technology can be expanded with the necessary system components on a modular basis so that they can be used flexibly for various products.